Details for: PAXLOVID
Company: PFIZER CANADA ULC
| Din | Din name | Active Ingredients | Strength | Dosage Form | Route of Adminstration |
|---|---|---|---|---|---|
| 02524031 | PAXLOVID | NIRMATRELVIR, RITONAVIR | 150 MG , 100 MG | Tablet | Oral |
| 02527804 | PAXLOVID | NIRMATRELVIR, RITONAVIR | 150 MG , 100 MG | Tablet | Oral |
Consumer Information
Information about the product including what the product is used for, dosage, warnings, proper use and side effects. This summary will not tell you everything about the product. Contact your healthcare professional if you have any questions about the product.
What the medication is used for
PAXLOVID is used in adults to treat mild to moderate coronavirus disease 2019 (COVID-19) in patients who:
- have a positive result from a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral test and
- who have a high risk of getting severe COVID-19, including hospitalization or death.
PAXLOVID IS NOT approved for any of the following:
- To treat patients who are hospitalized due to severe or critical COVID-19.
- To prevent COVID-19.
- To be used for longer than 5 days in a row.
- For use in children and adolescents less than 18 years of age.
What it does
COVID-19 is caused by a virus called a coronavirus. PAXLOVID contains two antiviral medicines co- packaged together, nirmatrelvir and ritonavir. PAXLOVID stops the virus from multiplying. This can help your body to overcome the virus infection and may help you get better faster.
When it should not be used
Do not use PAXLOVID if:
- You are allergic to nirmatrelvir, ritonavir or to any of the other ingredients in PAXLOVID (see What are the ingredients in PAXLOVID?).
You are taking any of the following medicines:
- alfuzosin, used to treat signs and symptoms of an enlarged prostate gland
- amiodarone, bepridil*, dronedarone, flecainide, propafenone, quinidine*, used to treat irregular heartbeats
- apalutamide, used for prostate cancer
- astemizole* or terfenadine*, used to relieve allergy symptoms
- carbamazepine, phenobarbital, primidone, phenytoin, typically used to treat seizures (epilepsy)
- cisapride*, used to relieve certain stomach problems
- colchicine, when used in patients with kidney and/or liver problems, used to treat gout
- eletriptan, ubrogepant, used to treat migraine
- eplerenone, ivabradine, used to treat heart failure and high blood pressure
- ergotamine*, dihydroergotamine (used to treat headaches), ergonovine, methylergonovine* (used after labour and delivery or abortion)
- finerenone, used to treat adults with chronic kidney disease and type 2 diabetes
- flibanserin, used to treat hypoactive sexual desire disorder in women
- fusidic acid, used as an antibiotic
- lovastatin, lomitapide or simvastatin, used to lower cholesterol
- lumacaftor/ivacaftor, used to treat cystic fibrosis
- lurasidone, pimozide, used to treat mental health problems
- naloxegol, used to treat constipation caused by narcotic pain medications
- neratinib, used to treat breast cancer
- ranolazine, used to treat chronic angina (chest pain)
- rifampin (used to treat tuberculosis) together with saquinavir/ritonavir (anti-HIV medication)
- rivaroxaban, used as an anticoagulant
- salmeterol, used for asthma and chronic obstructive pulmonary disease
- sildenafil, when used for the treatment of pulmonary arterial hypertension (PAH)
- silodosin, used to treat signs and symptoms of an enlarged prostate gland
- St. John’s Wort (Hypericum perforatum), an herbal product used to treat depression
- tolvaptan, used to treat low sodium in the blood
- triazolam and midazolam (oral* or injected), used to relieve anxiety and/or trouble sleeping
- PDE5 inhibitors vardenafil, used to treat erectile dysfunction
- venetoclax, used to treat chronic lymphocytic leukemia
- voriconazole, used as an antifungal
* Product is not or no longer marketed in Canada
What the medicinal ingredient is
Medicinal ingredients: nirmatrelvir, ritonavir.
What the non-medicinal ingredients are
Non-medicinal ingredients in nirmatrelvir: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, microcrystalline cellulose, and sodium stearyl fumarate. The film-coating contains hydroxy propyl methylcellulose, iron oxide red, polyethylene glycol and titanium dioxide.
Non-medicinal ingredients in ritonavir: anhydrous dibasic calcium phosphate, colloidal silicon dioxide, copovidone, sodium stearyl fumarate, and sorbitan monolaurate. The film-coating contains colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, polyethylene glycol, polysorbate 80, talc and titanium dioxide.
What dosage form it comes in
PAXLOVID consists of two medicines co-packaged together:
- Nirmatrelvir (pink tablet): 150 mg in each tablet
- Ritonavir (white tablet): 100 mg in each tablet
Paxlovid is supplied in two different Dose Packs. The Dose Packs differ in the number of nirmatrelvir tablets they contain. Your healthcare professional will prescribe the Dose Pack that is right for you.
Each Dose Pack contains the following:
Dose Packs |
Each Carton Contains |
Each Daily Blister Card Contains |
300 mg nirmatrelvir (as two 150 mg tablets); 100 mg ritonavir |
30 tablets divided in 5 daily- dose blister cards |
4 pink nirmatrelvir tablets (150 mg each) and 2 white ritonavir tablets (100 mg each) |
150 mg nirmatrelvir; 100 mg ritonavir |
20 tablets divided in 5 daily- dose blister cards |
2 pink nirmatrelvir tablets (150 mg each) and 2 white ritonavir tablets (100 mg each) |
Warnings and precautions
Serious Warnings and Precautions
Patients with kidney problems : Tell your healthcare professional before you take PAXLOVID if you have any kidney problems. You might need a lower dose of PAXLOVID. Your healthcare professional will prescribe a dose that is right for you.
To help avoid side effects and ensure proper use, talk to your healthcare professional before you take PAXLOVID. Talk about any health conditions or problems you may have, including if you:
- Have kidney problems
- Have liver problems including hepatitis
- Have human immunodeficiency virus (HIV) infection
Serious interactions with other medicines : Many medicines interact with PAXLOVID. Taking PAXLOVID with these medicines may cause serious or life-threatening side effects. Tell your healthcare professional about all the medicines you take before you start taking PAXLOVID. Do not take PAXLOVID if you are taking any of the medicines listed under the “Do not use PAXLOVID if:” section, below. Talk to your healthcare professional first before taking any new medicines. They will tell you if it is safe to take.
Other warnings you should know about:
Liver problems: Before you take PAXLOVID tell your healthcare professional if you have any liver problems. Liver problems have happened in patients taking ritonavir, a medicine in PAXLOVID. Talk to your healthcare professional if you get any symptoms of liver problems. These include: yellow skin or whites of eyes, nausea, tiredness or feeling unwell, loss of appetite, fever, skin rash, abdominal pain, pale stool or dark coloured urine.
Pregnancy and Birth Control: Tell your healthcare professional if you are pregnant, think you might be pregnant or are planning to become pregnant. You should not take PAXLOVID if you are pregnant unless your healthcare professional advises that you can. Women should use effective birth control while they are taking PAXLOVID. PAXLOVID may reduce the effectiveness of birth control pills, patches and vaginal rings. You should use an additional non-hormonal birth control method such as a condom, or spermicide while you are taking PAXLOVID. Continue to use the additional contraception method until your next period. Talk to your healthcare professional about effective methods of birth control.
Breastfeeding : Tell your healthcare professional if you are breastfeeding or plan to breastfeed. PAXLOVID can pass into your breastmilk. Your healthcare professional will tell you if you can breastfeed your baby while taking PAXLOVID.
Severe Allergic Reactions and Severe Skin Reactions: Before you take PAXLOVID tell your healthcare professional if you are allergic to nirmatrelvir, ritonavir or to any other ingredient in PAXLOVID. You must not use PAXLOVID if you are allergic to any of its ingredients. Allergic reactions have happened in patients taking PAXLOVID, even after only one dose. These include skin rash, hives, itching of the skin, swelling under the skin, swelling of the mouth, lips, tongue, face, and extremities, swelling and tightness of the throat, hoarseness, low blood pressure, fainting, weakness, difficulty in swallowing or breathing. If you experience signs of a severe allergic reaction and / or severe skin reactions, you should immediately stop taking PAXLOVID and consult your healthcare professional.
Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have happened in patients taking ritonavir, a medicine in PAXLOVID. These are severe skin rashes / reactions. The signs of Stevens Johnson syndrome (SJS) include redness, blistering and/or peeling of the skin and/or inside of the lips, eyes, mouth, nasal passages or genitals, accompanied by fever, chills, headache, cough, body aches or swelling and the signs of toxic epidermal necrolysis (TEN) include redness, blistering and/or peeling of large areas of the skin. If you experience signs of Stevens Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN), you should immediately stop taking PAXLOVID and consult your healthcare professional.
Cholesterol-lowering medicines: Before you take PAXLOVID tell your healthcare professional if you are taking a cholesterol-lowering medicine such as lovastatin or simvastatin. PAXLOVID may increase the amount of these medicines in your body. You should stop taking lovastatin / simvastatin at least 12 hours prior to starting PAXLOVID. You must not take these while you are taking PAXLOVID and for 5 days after stopping treatment.
Interactions with this medication
Tell your healthcare professional about all the medicines you take, including any drugs, vitamins, minerals, natural supplements or alternative medicines.
Do not take PAXLOVID if you are taking any of the medicines listed under the “Do not use PAXLOVID if:” section. Taking PAXLOVID with these medicines may cause serious or life-threatening side effects.
The following may also interact with PAXLOVID:
- medicines used to treat erectile dysfunction, such as sildenafil, and tadalafil
- medicines used to treat pulmonary arterial hypertension, such as bosentan, riociguat or tadalafil
- medicines used to lower blood cholesterol, such as atorvastatin and rosuvastatin
- some medicines affecting the immune system, such as cyclosporin, sirolimus and tacrolimus
- some medicines used to treat seasonal allergies and ear and eye infections, such as budesonide, dexamethasone, fluticasone propionate, prednisone, and triamcinolone
- medicines used to treat HIV and related infections, such as amprenavir, bictegravir, efavirenz, indinavir*, nelfinavir, saquinavir, didanosine*, rifabutin, tipranavir, delavirdine*, atazanavir, maraviroc, fosamprenavir, raltegravir, tenofovir, nevirapine, zidovudine emtricitabine and darunavir
- medicines used to treat depression, such as trazodone, desipramine and bupropion
- certain heart medicines, such as calcium channel antagonists including amlodipine, diltiazem, felodipine, nicardipine, nifedipine and verapamil
- medicines used to treat men with symptoms of an enlarged prostate such as tamsulosin
- medicines used to correct heart rhythm, such as systemic digoxin and lidocaine, disopyramide and mexiletine
- medicines used to treat heart or blood vessel problems such as aliskiren, and vorapaxar
- certain blood thinners such as apixaban, clopidogrel, dabigatran, ticagrelor and warfarin
- antifungals, such as ketoconazole, isavuconazonium sulfate and itraconazole
- morphine-like medicines used to treat severe pain, such as methadone and meperidine
- certain antibiotics, such as rifabutin, erythromycin and clarithromycin
- antibiotics used in the treatment of tuberculosis, such as rifampin
- bronchodilators used to treat asthma, such as theophylline
- medicines used to treat cancer, such as abemaciclib, ceritinib, dasatinib, encorafenib, ibrutinib, ivosidenib, nilotinib,vincristine and vinblastine
- medicines used for low blood platelet count, such as fostamatinib
- some anticonvulsants, such as clonazepam, divalproex, lamotrigine and ethosuximide
- some narcotic analgesics, such as fentanyl in all forms, hydrocodone, oxycodone, meperidine, tramadol and propoxyphene
- quetiapine used to treat schizophrenia, bipolar disorder and major depressive disorder
- medicines used to treat hepatitis C, such as simeprevir, glecaprevir/pibrentasvir, ombitasvir/paritaprevir and ritonavir with or without dasabuvir*, elbasvir/grazoprevir, and sofosbuvir/velpatasvir/voxilaprevir
- some sedatives or medicines to treat anxiety, such as buspirone, clorazepate, diazepam, flurazepam and zolpidem
- stimulants, such as methamphetamine
- medicines used to treat pain associated with endometriosis, such as elagolix
- medicines used to treat depression, such as amitriptyline, clomipramine, fluoxetine, imipramine, maprotiline*, nefazodone*, nortriptyline, paroxetine, sertraline, trimipramine, venlafaxine
- medicines used to treat nausea and vomiting, such as dronabinol*
- medicines used to treat pneumonia, such as atovaquone
- medicines used as a sedative and medicines used to help you sleep (hypnotics), such as estazolam*
- medicines used to treat increased pressure in the eye, such as timolol
- medicines used to lower blood pressure, such as metoprolol
- medicines used to prevent organ rejection after a transplant, such as everolimus, rapamycin*
- medicines used to treat certain mental health and mood disorders (including schizophrenia and bipolar disorder), such as aripiprazole, brexpiprazole, cariprazine, clozapine, iloperidone*, lumateperone*, perphenazine, pimavanserin*, risperidone, suvorexant*, and thioridazine*
- medicines used as hormonal contraceptives containing ethinyl estradiol (“the pill”)
- medicines used to treat cystic fibrosis such as ivacaftor, elexacaftor/tezacaftor/ivacaftor and tezacaftor/ivacaftor
- medicines used to control blood sugar levels such as, saxagliptin
- medicines used to treat rheumatoid arthritis and psoriatic arthritis such as tofacitinib and upadacitinib
- medicines used to treat malaria such as quinine
- medicines used to treat symptoms of an overactive bladder such as darifenacin
*Product is not or no longer marketed in Canada.
Proper use of this medication
How to take PAXLOVID:
- PAXLOVID consists of two medicines co-packaged together:
- nirmatrelvir (pink tablet)
- ritonavir (white tablet)
You must always take the nirmatrelvir tablet(s) at the same time as the ritonavir tablet.
- Always take PAXLOVID exactly as your healthcare professional has told you to.
- Check with your healthcare professional if you are not sure.
- You can take PAXLOVID with or without food.
- Swallow the tablets whole. Do not break, chew or crush the tablets.
- You must take PAXLOVID for 5 days in a row. Complete the entire 5-day treatment with PAXLOVID.
- Even if you feel better, do not stop taking PAXLOVID without talking to your healthcare professional first.
- Talk to your doctor if you do not feel better or if you feel worse after 5 days.
- If you have kidney problems, talk to your healthcare professional. You may need to take a lower dose.
Usual dose:
Adults:
Take 2 pink nirmatrelvir tablets and 1 white ritonavir tablet. Take these 3 tablets at the same time, twice a day (in the morning and again in the evening) for 5 days.
Each daily blister card shows your morning and evening dose, as follows:

Dose for patients with moderate kidney impairment:
If you have kidney problems, talk to your healthcare professional. You may need to take a lower dose.
Adults with moderate kidney impairment:
Take 1 pink nirmatrelvir tablet and 1 white ritonavir tablet. Take both tablets at the same time, twice a day (in the morning and again in the evening) for 5 days.
Each daily blister card shows your morning and evening dose, as follows:
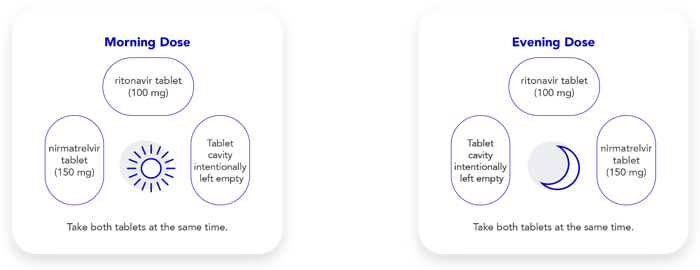
Overdose:
If you think you, or a person you are caring for, have taken too much PAXLOVID, contact a healthcare professional, hospital emergency department, or regional poison control centre immediately, even if there are no symptoms.
Missed Dose:
If you miss taking your dose and it:
- is within 8 hours of the time it is usually taken, take it as soon as you remember.
- has been more than 8 hours , skip the missed dose and take the next dose at your regular time. Do not take 2 doses of PAXLOVID at the same time.
Side effects and what to do about them
These are not all the possible side effects you may have when taking PAXLOVID. If you experience any side effects not listed here, tell your healthcare professional.
Side effects may include:
- altered sense of taste
- diarrhea
- muscle pain
- vomiting
- high blood pressure
- headache
- abdominal pain
- nausea
- general feeling of discomfort
Not many people have taken PAXLOVID. Serious and unexpected side effects may happen. PAXLOVID is still being studied, so it is possible that all the side effects are not known at this time.
Serious side effects and what to do about them |
|||
Talk to your healthcare professional |
Stop taking drug and get immediate medical help |
||
In all cases |
|||
Only if severe |
|||
Common |
|||
High blood pressure |
X |
||
Severe allergic reaction: skin rash, hives, itching of the skin, swelling under the skin, swelling of the mouth, lips, tongue, face and extremities, swelling and tightness of the throat, hoarseness, low blood pressure, fainting, weakness, difficulty in swallowing or breathing. |
X |
||
Rare |
|||
Stevens-Johnson syndrome (SJS) (severe skin rash): redness, blistering and/or peeling of the skin and/or inside of the lips, eyes, mouth, nasal passages or genitals, accompanied by fever, chills, headache, cough, body aches or swelling. |
X |
||
Toxic Epidermal Necrolysis (TEN) (severe skin reaction): redness, blistering and/or peeling of large areas of the skin. |
X |
||
Liver problems: yellow skin or whites of eyes, nausea, tiredness, loss of appetite, fever, skin rash, abdominal pain, pale stool, or dark coloured urine. |
X |
||
Symptom / effect
If you have a troublesome symptom or side effect that is not listed here or becomes bad enough to interfere with your daily activities, tell your healthcare professional.
How to store
Store at room temperature 15°C to 30°C. Keep out of reach and sight of children.
Reporting side effects
Reporting Side Effects
You can report any suspected side effects associated with the use of health products to Health Canada by:
- Visiting the Web page on Adverse Reaction Reporting (https://www.canada.ca/en/health- canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting.html) for information on how to report online, by mail or by fax; or
- Calling toll-free at 1-866-234-2345.
NOTE: Contact your health professional if you need information about how to manage your side effects. The Canada Vigilance Program does not provide medical advice.
More information
If you want more information about PAXLOVID:
- Talk to your healthcare professional.
- Find the full product monograph that is prepared for healthcare professionals and includes this Patient Medication Information by visiting the Health Canada website: (https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug- product-database.html; the manufacturer’s website www.pfizer.ca, or by calling 1-800-463-6001.
This leaflet was prepared by Pfizer Canada ULC.
Last Revised June 27, 2023